Musfeldt Leverages Pressure for Future Energy Storage
A group of researchers including Jan Musfeldt, Ziegler professor of chemistry at the University of Tennessee, recently published in the Proceedings of the National Academy of Sciences (PNAS). The article details the discovery that pressure can be used to create and control the phase and properties of hafnia.
Hafnium oxide, or hafnia, is a material that has interested researchers for many decades. Highly insoluble and temperature stable, hafnia has been leveraged in applications as diverse as optical coatings for glasses and camera lenses, fuel cells, and passive building insulation. Advanced electronics and energy storage applications are, however, generating the most excitement.
Since 2007, amorphous hafnia thin films have been employed as gate dielectrics in CMOS technology, enabling the continuation of Moore’s scaling of DRAM chips. Ferroelectricity and silicon compatibility make hafnia an attractive candidate for nonvolatile ferroelectric FET devices as well as negative capacitance heterostructures. Recent break-throughs impacting the development of advanced chip technologies include the discovery of flat phonon bands and their connection to the energy landscape in the vicinity of the polar phase and the incredibly rich variety of competing phases in crystalline thin films of hafnia.
“I love this project!” said Musfeldt. “It brings together the fundamental science of phase competition under external stimuli and work at the Frontier Infrared Spectroscopy beamline at the National Synchrotron Light Source II with the growth of whole families of high-quality single crystals and complementary first-principles theory to predict phase stability, signatures, and properties. It’s also a great chance to work with friends from Rutgers, Rochester, and Brookhaven.”
One challenge in this field involves the use of very high temperature laser floating zone and rapid quenching process of the crystal growth combined with an yttrium stabilizer in concentrations of up to 20%. While effective, the high levels of yttrium used impacts the purity of the material, which in turn affects performance.
Musfeldt and her collaborators wanted to investigate an alternative means of creating the various phases of hafnia using less stabilizer. They settled on compression (or pressure) as a technique with the potential to do the job. The fact that pressure changes bond lengths and angles provides access to exciting new and stable phases of materials. A similar approach was used in the development of synthetic diamonds.
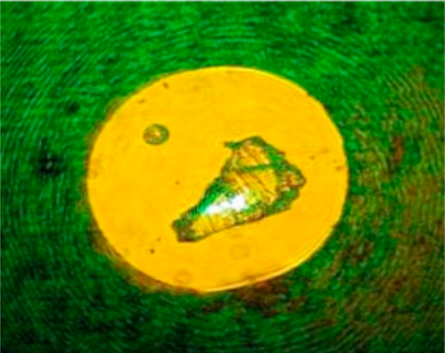
Hafnia crystal photographed by Jan Musfeldt.
Musfeldt’s team, including post-doctoral researcher Yanhong Gu, conducted a series of experiments using pressure to coax hafnia into the desired antiferroelectric phase. They found that not only did this method work, but the material also remained stable once pressure was removed. Repeated analysis of the product led them to conclude that the material was indefinitely stable at room temperature.
“The material remains stable both with time and through high-temperature annealing,” said Gu.
In addition to showing that compression was a suitable means of achieving a stable antiferroelectric state in hafnia, Musfeldt’s team also confirmed this “pressure-induced chemical reaction” could be done at room temperature with 36% less yttrium stabilizer than previously required by high temperature growth techniques.
“Reducing the among of yttrium stabilizer is crucial to preserving the fundamental performance of these materials”, says Musfeldt.
At the same time, the team was able to use even higher pressures to create the tetragonal form of hafnia. This phase had been theoretically predicted to exist but never confirmed until now.
To identify these previously elusive phases, Musfeldt’s team used predictions developed by her collaborators at the University of Rochester and Rutgers University. Sobhit Singh, David Vanderbilt, and Karin Rabe specialize in computationally predicting the properties of materials. They calculated that compression could be used to stabilize both the antiferroelectric and tetragonal forms of hafnia as well as the vibrational fingerprints that were matched with the experimental measurements.
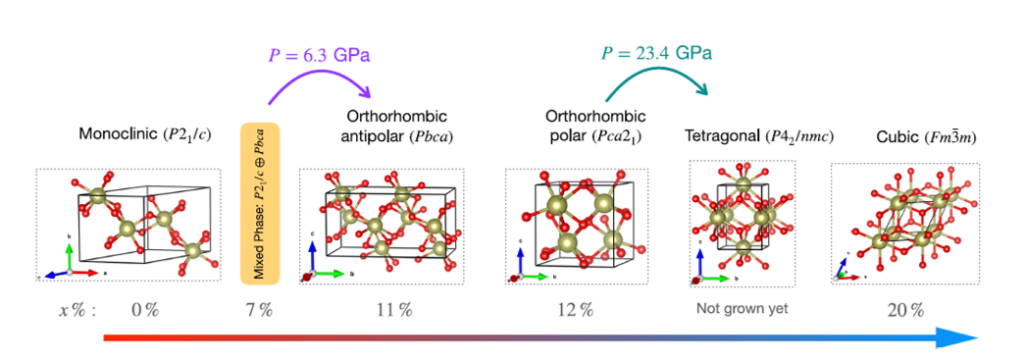
This figure from the publication summarizes the pressure pathways and various phases of hafnia, including the theorized tetragonal phase, confirmed by Musfeldt.
“Our first-principles calculations unambiguously predict the phase transition from the mixed-phase structure to the desired pure-metastable phase of hafnia at pressures above 6 GPa,” said Sobhit Singh, co-author and assistant professor at the University of Rochester.
“It’s exciting when theory and experiment dovetail so beautifully”, says Musfeldt.
PNAS is one of the world’s most-cited and comprehensive multidisciplinary journals. The full article can be found here.
Feature image courtesy of Sobhit Singh, University of Rochester.