Jenkins Group Published in Langmuir and Chemical Science
The Jenkins Group published their work “Imidazolinium N-Heterocyclic Carbene Ligands for Enhanced Stability on Gold Surfaces” in Langmuir. This work explores the preparation and stability of NHC-coated gold surfaces using imidazolium and imidazolinium NHC ligands. X-ray photoelectron spectroscopy and surface-enhanced Raman spectroscopy demonstrate the attachment of NHC ligands to the gold surface and show enhanced stability of imidazolinium compared to the traditional imidazolium under harsh acidic conditions.
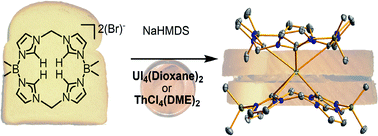
The Jenkins Group also published their work “Actinide tetra-N-heterocyclic carbene ‘sandwiches’” in Chemical Science. “We synthesized new “sandwich” complexes by placing two NHC macrocycles around a single actinide ion,” Jenkins said. “I am particularly excited about this paper since it is work that I began on my sabbatical at the University of Edinburgh almost four years ago. It is the beginning of a new research area in my group, which is f-block NHC chemistry.”