Rising Scholars: Dylan Andrews
Some students begin their college careers knowing they want a good education but unsure about what comes next, while others move in to their dorms with the next steps toward their career firmly in mind.
Dylan Andrews, senior honors chemistry major, was one of the latter. A native Tennessean, Andrews came to the University of Tennessee, Knoxville in pursuit of an education that would ultimately get him to medical school, starting with an undergraduate degree in chemistry.
“I was fortunate enough to have a really amazing chemistry instructor in high school, Mr. Mark Page. He was one of those teachers who truly makes an impact on you and he really helped me develop a love for chemistry,” said Andrews.
As he pursued his degree at UT, Andrews began to see participating in research as an opportunity to make the most of his time at the university and better prepare himself for the future. He broached the topic with Professor Janice Musfeldt, who was teaching one of his classes at the time.
“I think this is a really good example of how students can get involved in research in the department. Dr. Musfeldt and I built a good relationship over the course of the semester. I also met one of her graduate students and attended a seminar delivered by her colleague, Hans Bechtel. This let me get to know her group and her research, while showing her that I was engaged and interested,” said Andrews.
Hans Bechtel is the infrared program lead for the Advanced Light Source (ALS) at Lawrence Berkeley National Laboratory. His ongoing relationship with the Musfeldt Group has led to him co-authoring several publications with its members. Bechtel visited the university to deliver a seminar and, over the course of conversation afterwards, suggested Andrews apply for a Department of Energy (DOE) summer internship at the Lawrence Berkeley Lab later in the year.
The next semester Andrews embraced research in the chemistry department as the next step toward his goals. He registered for the undergraduate research course and joined the Musfeldt lab. Heeding Bechtel’s advice, Andrews also applied for and was awarded a place in the DOE summer program at Lawrence Berkeley.
Near the end of spring semester, Andrews participated in the Department of Chemistry’s annual Undergraduate Research Symposium, presenting a poster to a panel of judges including departmental alumni, retired faculty, and industry partners. This experience gave Andrews his first chance to speak publicly about his research; an opportunity that would pave the way for future poster presentations.
At the end of his internship at Lawrence Berkeley Lab, Andrews entered and placed third in a poster competition designed to evaluate the presentation skills of the participants. The presentations were conducted via Zoom, allowing members of Andrews’ research team in the Musfeldt Group to join and support him.
Andrews plans to graduate in December 2024 and go on to medical school. He believes his experience in the Department of Chemistry and the relationships forged there have prepared him to meet the challenges of a future in medicine.
“Dr. Musfeldt, and really every faculty member I’ve worked with in the department, do everything they can to plug their students into new opportunities and point out things they could do to better themselves as students and researchers. I would probably never have known about that DOE internship if I hadn’t been introduced to Dr. Bechtel,” said Andrews. “The relationships I’ve developed and the support I’ve experienced in the chemistry department have really helped me excel as a student, which will help me through all the next stages of my education and career.”


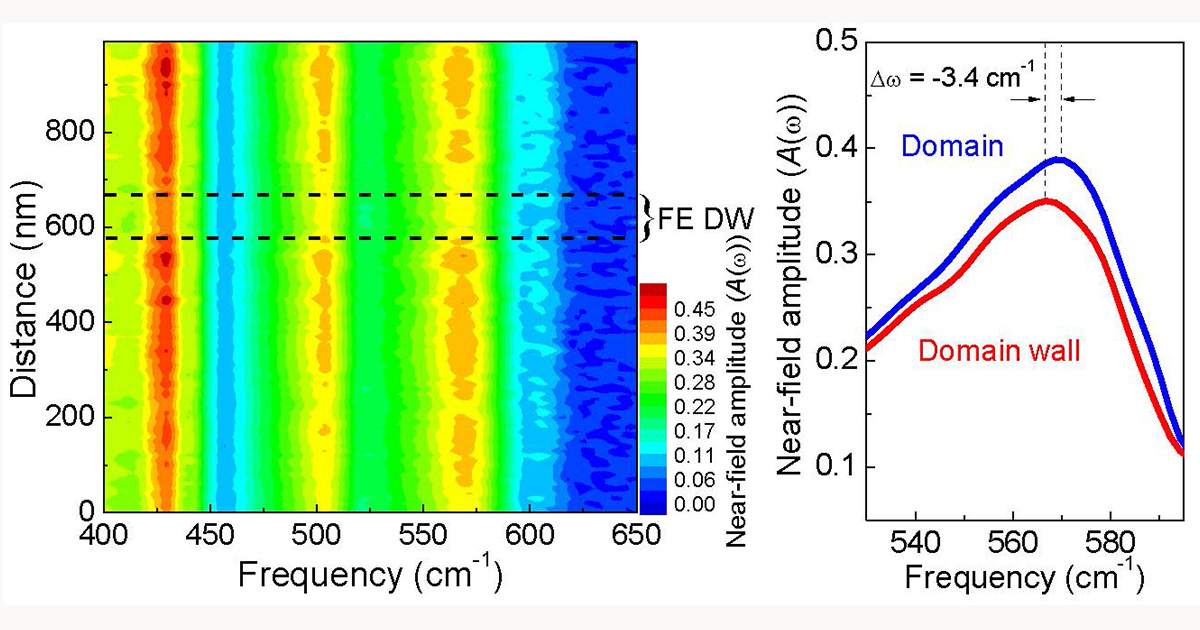
 Smith joined the chemistry department as a graduate student in 2015 and very quickly began investigating domain walls. Domain walls act as the boundaries between regions, or domains, of materials and have the potential to impact the properties and uses of that material.
Smith joined the chemistry department as a graduate student in 2015 and very quickly began investigating domain walls. Domain walls act as the boundaries between regions, or domains, of materials and have the potential to impact the properties and uses of that material. “This project really highlights the importance of curiosity in research,” said Musfeldt. “Kevin took an exploratory project and turned it into the most exciting thing in our lab with far-reaching implications.”
“This project really highlights the importance of curiosity in research,” said Musfeldt. “Kevin took an exploratory project and turned it into the most exciting thing in our lab with far-reaching implications.”